|
UV Fluorescence
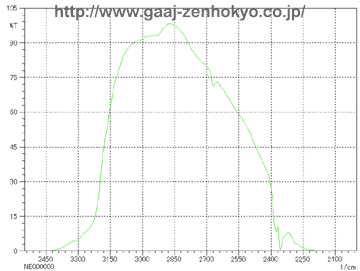
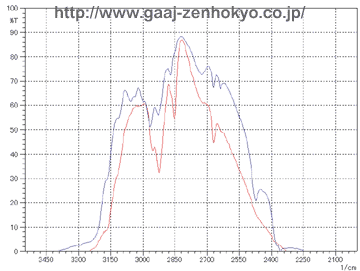
Emeralds will show red fluorescence due to chromium according to their localities, and they will show no fluorescence (inert) if they contain impurity elements such as iron. When resin is filled stones may fluoresce in blueish white to faint blueish white colour. Emerald filled with oil sometimes show bright yellow-green fluorescence. Depends on the type of filling material, however, many stones do not show any fluorescence, and only characteristic fluorescence if observed can be a help of identification. FTIR Spectra Analysis FTIR stands for Fourier Transformation Infra-Red, and it is a spectrophotometer in infrared region that can obtain absorption spectra with frequency analysis called Fourier Transformation on a waveform, which is obtained from a sample by illuminating it with a composite waveform that is made from light from a light source through an interferometer. By obtaining molecular vibration in a substance, FTIR can effectively analyse water, hydroxyl and resin or oil in crystals. On the FTIR spectrophotometer an emerald is generally measured by dispersive reflection mode with its table facet down on a coating film mirror (sample stage). A light entering from the pavilion of the stone passes through the stone and is reflected at the mirror, back into the stone again and goes into a detector. In this way information of a whole stone can be observed by FTIR analysis and there is no need to locate a fissure position beforehand. However a sample to be measured should be rotated through 360on the mirror because fissures filled with resin or oil consist only small part of a stone and an incident light should pass through the filled area. @@FTIR measurement shows large absorption band of emerald itself, and a spectrum of filled substance must be detected in a limited range of transmission band (3200`2400cm-1). FTIR spectra of untreated emerald and that of emerald filled with resin and oil are show in figure 1 and 2 respectively.
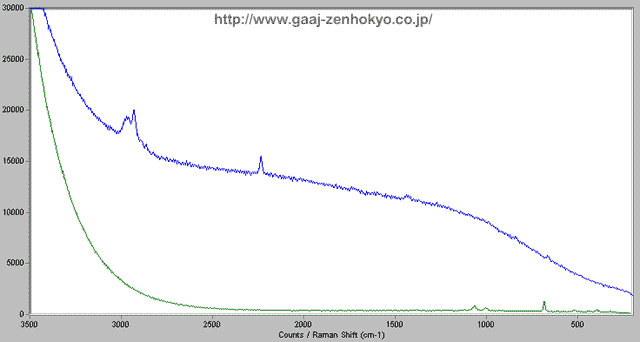
Microscopic Raman Spectroscopy When a light is hit a substance and scattered, some of the scattered light may change its wavelength by a certain amount that is unique to each substance. This phenomenon is named Raman Effect after the discoverer. Raman spectroscopy is a technique to identify a substance or to analyse molecular structure by using the phenomenon. The scattering light obtained by Raman Effect is generally very weak so that a strong laser beam is used for irradiation and a sophisticated detector is required. Raman spectroscopy with a microscope has high spatial resolution and it can be used for analyses in local or minor areas, and also for inclusion inside gems if it is close to the surface. In detection of filling material in emerald, local analysis can be performed by focusing on a possibly-filled fissure under magnification with a microscope. Figure 3 shows examples of detection on resin filled in a fissure.
<< Summary >> Observation of various characteristic features under a gemmological microscope is very important to detect filling in emerald. Flash effect observed under a certain condition with illumination techniques is a feature of filling with a substance such as resin that has close RI to emerald. Existence of flat gas bubble or altered (turned brownish or whitish) residue will be a clue of filling. Characteristic fluorescent colour such as blueish white or yellow-green under UV light may originate in a filling substance. FTIR spectrophotometry is effective to detect filled resins or oils because it can obtain molecular vibration of a substance. With this method the whole stone can be analysed and the quantity of filling substance (i.e., degree of filling) can be roughly estimated. Raman spectroscopy has high spatial resolution, and a fissure that is suspected to have been filled can be analysed by spotting it under magnification with a microscope. |
||||||||||||
|