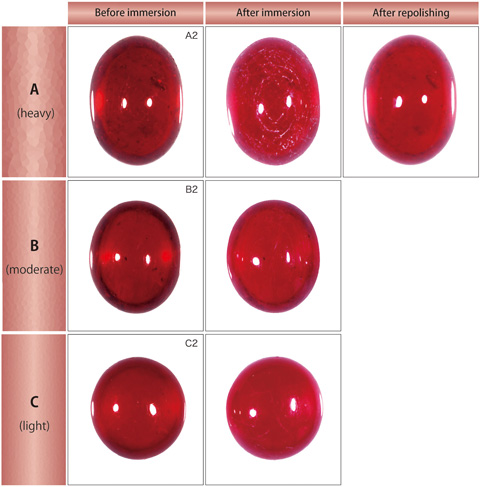
| Test results ?EResistance to pickling solution One piece each from the three groups was picked out and soaked into a solution (pickling compound ?Emain ingredients; acid sodium sulphite Na2HSO3 ?Ediluted with a defined amount of warm water about 50?E that is generally used for acid pickling in manufacturing or repairing jewellery, and they were observed change in their appearance or surface conditions. Immersing period was set for 5 minutes, at the longest estimate in a common manufacturing process. The lead-glass on the surface started to be dissolved immediately after the immersion and it appeared as white streaks. The stones were then taken out from the solution and observed. Although weight loss was not recognised, lead glass filled fissures on the surface were corroded and became obvious as grooves (figure 6). ?? Meanwhile, a ruby with corroded surface in such a manner can retrieve the appearance by repolishing. The most severely corroded one of group A recovered to the almost original appearance after repolishing with about 2 % loss of the weight (figure 6).
|
||||||||||||||||||||
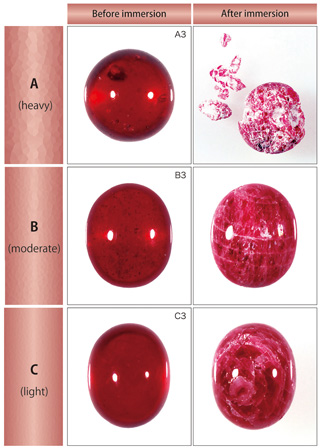
| ?EResistance to hydrofluoric acid One piece each from the three groups was picked out and soaked into a hydrofluoric acid solution (hydrofluoric acid concentration 46 %). Hydrofluoric acid has property to corrode glass and this was applied to this test to investigate any change of weight or appearance of ruby that were immersed in the solution to dissolve the filled lead-glass. ?? The lead-glass dissolved after immersion as time progresses, and precipitation of white residues were recognised (figure 7). The stones were taken out from the solution three days later and dried for observation (figure 8). The fractures, from which the lead-glass was slipped out, appeared whitish (figure 9) and this proved that the filling had vastly improved the stone clarity. A stone with filling in heavy degree shows more white areas spreading throughout the stone, which indicates that the original stone had many fractures and twin planes (figure 8). Ruby samples in the group A that were the most severely filled were partly crumbled and this is highly possibly caused by small pieces coming off from densely contained fractures or twin planes that cracked when lead-glass dissolved.
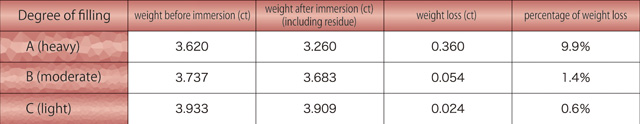
?? Ruby samples mainly of group A and B had white residues on the surface. These stones showed weight loss from 0.6 to 9.9 % after the test even with such residues (table 1). Considering the residues, the weight actually gained by the lead-glass filling is presumably larger than this.
|
||||||||||||||||||||
| Summary We obtained lead glass filled ruby currently circulated on the market to examine changes in appearance or weight by immersion test with acids. As the result, stone surface was slightly corroded by immersion in a solution that is commonly used for acid pickling in manufacturing or repairing jewellery. However, such corroded surface can be recovered by minimal repolishing. Distinct weight loss was also recognised after immersion test with hydrofluoric acid solution that dissolved the lead-glass, and the amount of weight loss was as much as 10 % in the most severely filled stone. From the results above it is confirmed that lead glass filled ruby gains its weight by filling treatment and it is vulnerable to acids. Ruby filled with lead-glass in various degrees is currently circulating in the market and care should be taken when dealing with ruby. For further information, please contact: GAAJ-ZENHOKYO Laboratory E-mail: laboratory@gaaj-zenhokyo.co.jp |
||||||||||||||||||||
|
|