| üč Standard Identification Tests ü× Appearance A rough crystal of CVD diamond synthesized by Apollo Diamond inc. has totally different appearance from a rough crystal of natural diamond. Most of natural diamonds show crystal forms based on an octahedron, while CVD diamonds show tabular form (Fig 2). Those crystal forms are also different from cube-octahedral form of previous HPHT synthetic diamond. Two pieces of rough stones tested this time were both completely separated from their substrates (Apollo Diamond inc. uses HPHT synthetic diamond for their substrates, which are removed by laser after the CVD crystal growth.). Outer rim of the rough crystals show dark brown area of low quality, which is non-diamond carbon (Photo 3/ front page). Two pieces of Element Six and one piece of Apollo Diamond inc. were faceted, with the latter have been HPHT treated and thus its transparency was lowered and facet surface was roughened (Photo 4/ front page). According to Mr. Branko Deljanin of EGL, brown tint of the stone has been slightly removed by the HPHT treatment. ü× Inclusion CVD diamonds tested this time were generally good in clarity, and two faceted pieces of Element Six were in VS class*. A few pinpoints were observed under 10X magnification in a tabular polished stone of Apollo Diamond inc. (Photo 5). Further magnifying observation revealed that those pinpoints were dark brown irregular-shaped substance, which is though to be non-diamond carbon. As CVD synthesis does not involve metal solution, metal inclusion, which is often seen in synthetic diamond produced by HPHT process, will not exist. Therefore, CVD diamond does not have magnetism, which has been announced as one of the simple identifying features for synthetic diamonds.
ü× Colour Zoning All CVD diamonds tested this time except one piece, which is near colourless of Element Six, have a brown hue. They are graded as Fancy Light Brown and Fancy Brown*, with a slight yellow hue. Brown colour distribution in the CVD diamonds was observed evenly throughout each stone, however some stones showed several brown lines. These are probably parallel to {100} and may have been caused by deposition of non-diamond carbon due to variation of temperature or gas pressure during its growth. Brown colour zoning seen in natural diamonds has been formed by plastic deformation that the diamond received while it reached to the earthüfs surface from deep under the ground where it had originally formed. It is usually observed as brown grains (planete brown colour zoning) arranged in one direction or two directions intersecting to one another. The brown line pattern seen in CVD diamond appears not being related to anomalous double refraction due to strain that is going to be mentioned later, while the brown colour zoning seen in natural diamond is commonly associated with the anomalous double refraction showing interference colour. ü× Anomalous Double Refraction due to Strain The CVD diamonds we tested this time showed characteristic streaky pattern of anomalous double refraction due to strain. They run in parallel to the growth direction of the crystal, which can be clearly observed from the direction perpendicular to the growth plane of the tabular crystal (i.e., mainly from the girdle). These patterns are thought due to dislocation caused during growth of the crystal (Photo 6). Anomalous double refraction due to strain seen in natural diamond can be roughly devided into two categories, one of which is caused during the growth and the other due to plastic deformation, the latter strongly indicates natural origin of the stone. Typical anomalous double refraction due to strain caused by plastic deformation is so-called üeTatamiüf structure seen in a type II diamond (Photo 7). Although CVD diamond belongs to this type II, it does not show the üeTatamiüf structure. However, when a CVD diamond is observed from the direction parallel to the growth direction (usually along the direction perpendicular to the table), the stone shows üeTatamiüf -like structure on first glance so that care should be taken (Photo 8) not to be deceived.
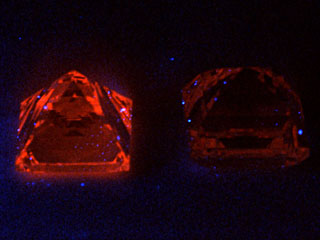
ü× UV Fluorescence Most pieces of the CVD diamonds tested this time showed characteristic orange UV fluorescence (Photo 9). They were generally more clearer in LWUV than in SWUV, but if intensity of the fluorescence is weak, it should be observed in a completely dark room. Those fluorescence colour is thought due to NV centre (575nm), which is rarely seen in a type II natural diamond. In CVD diamond, injection of nitrogen gas to accelerate growth rate and formation of void associated with generation of dislocation may be involved in production of the NV centre.
üč Laboratory Techniques ü× UV-Visible Spectrum Spectral analysis between UV-visible region under room temperature showed gradually stronger absorption from LWUV towards SWUV. The absorption end in the UV region is between 220nm and 240nm, which is a typical spectrum pattern of type II. In samples with a brown hue, broad absorptions were observed between 500nm and 550nm. The intensity of absorption and the depth of the brown hue are in proportion, and this absorption was not seen in a near colourless sample. Some stones showed a slight absorption on 270nm, which is due to isolated single atoms of nitrogen. N3 centre (415nm), which is seen in almost all natural diamonds, was not recognised. The Diamond Trading Companyüfs Diamond Verification Instruments- DiamondSure is also a very convenient technique to detect the N3 centre as a factor to determine the type of diamond. Natural Type Ia diamond indicates as ügpassüh and various Type II as ügreferüh, it means that the CVD synthetic diamond will be certainly refer and further testing is required. ü× Infrared Spectrometry (FTIR) Spectral analysis in near-infrared to infrared region under room temperature was examined, and in the infrared region, most of the samples were in the type II category which does not show absorption in nitrogen region in diamond. Some samples showed a very weak absorption on 1344cm-1 when the resolution power was set to 1cm-1 and its elapsed time was increased. This is related to isolated single atom of nitrogen and is typically seen in type Ib. In the near-infrared region, some showed a slight absorption on 7354cm-1. According to Wuyi Wang et.al, (2003), CVD diamonds may show absorptions on 8753, 6856, 6425 and 5564cm-1 relating to hydrogen impurity when they are analysed with elapsed time of 1024. |
|||||||||||||||||||||
|