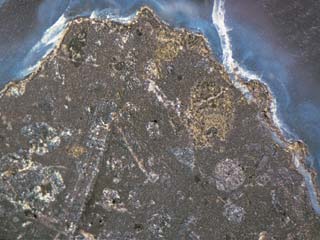
| Chemical Compositional Analysis X-ray Fluorescence Analysis and LA-ICP-MS system Similarly to agate, chalcedony can be easily artificially dyed with pigment because numerous minute voids exist inside the material. The stone tested this time proposes unusual colour and we analysed the piece by X-ray fluorescency to confirm the cause of the colour. As shown in the Table 2, elements such as Fe and Ca were detected as trace elements other than Si, the main element of chalcedony, and the content of Fe2O3 was 0.04ü`0.16%. After measurement of trace elements by LA-ICP-MS, ultra trace elements in tens to several ppm order such as Ti, Ba, B, Mn, Sn, Ga and Be (in ascending order) were detected, however, common colouring agent such as Cr, V, Co and Ni were under detection limit. This concludes that the violet colour of the chalcedony piece is mainly derived from iron content possibly with influence of some Ti and Mn. The host rock containing the chalcedony is black and partly eroded by weathering (Photo 4). Idiomorphic olivine, pyroxene and plagioclase are recognized under a microscope observation. The average chemical composition of the host rock was SiO4 47% and over ten percents of Al2O3, CaO and Fe2O3 (FeO), with several percents of Na2O, MgO and TiO2 by ED-XRF analysis (Table 2). Trace elements such as K, Zn and Sr were also detected, and the stone is petrologically classified as alkali basalt.
Colour Fading Test and Irradiation Test In general, chalcedony may fade its colour by light or heat so that we performed a fading test. The stone was exposed to a 150w halogen light about 10cm apart from the source for more than ten hours to keep the temperature at 100ºC. The colour obviously became paler than the one at the identification (Photo 5). The faded chalcedony was then irradiated with ā┴-ray, at the Tokyo Metropolitan Industrial Technology Research Institute in the cobalt irradiation chamber 1 (hot cell) in distance of about 5cm from the source for 18 hours. The total dose was 1.7ü~107 roentgen. After the irradiation, the stone recovered the same violet colour as it was (Photo 6). Such cycle of fading-recovering of colour is fairly similar to that seen in amethyst. From our chemical analyses and the condition of the host rock, the colour cause of this violet chalcedony is presumed to be a colour centre relating iron that is similar to amethyst.
Summary A violet chalcedony from Indonesia consisting of minute quartz aggregates occurs as masses or veins in alkali basalt and its violet colour is produced mainly by iron (Max; 0.16% Fe2O3). The stone has an even structure with a little impurity throughout the crystal, with characteristic partial distribution of agate-type colour zoning. LA-ICP-MS analysis on ultra trace elements detected tens to several ppm order of Ti, Ba, B, Mn, Sn, Ga and Be, but no common colouring agent was detected. If this kind of violet chalcedony can be supplied in sufficient amount in the future, it will add a new line to gem chalcedony. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||