|
||||||||||||||||||||||||||||||||
|
Recently one beads necklace shown in the photo-1 was brought into our laboratory for identification. The stones appeared just as traditionally-known blue aragonite from Peru (Gems & Gemology Winter 1992, Gem News) at a glance; however, our inspection revealed that the necklace consisted of mixture of natural and dyed aragonite. The geographic origin of the stones were not specified this time.
The stones were white beads with blue and green spots in almost uniformed 8 mm size and they had dull vitreous lustre. The surface and interior were observed under magnification and three types of surface structure were recognised in the series of the beads.
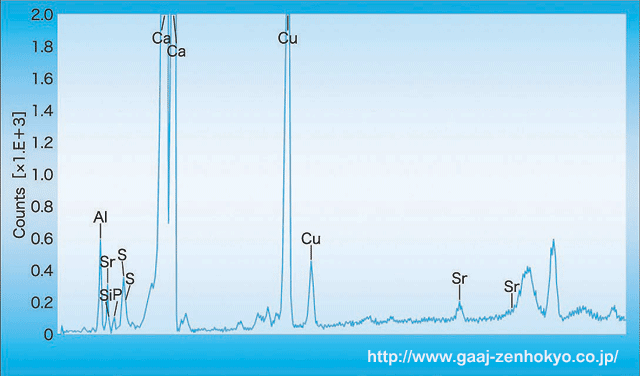
RI measurement by spot method ranged between 1.60 and 1.65, with DR tending to large. SG measured as 2.75, which was lower than the standard value for aragonite (2.94). All the beads showed faint blue-white colour under LWUV and they were inert under SWUV. No change was observed through a colour filter, and no characteristic absorption was observed on a hand-held spectroscope. In the chemical compositional analysis by EDXRF (fluorescent X-ray spectral analysis) more than 99 wt% in the Type-1 colourless-white radial fibrous crystal beads was main component CaO. Other than that, P and Sr were detected in very small amount, therefore this aragonite composition was confirmed to be in high purity. In the Type-2, white crystalline beads containing blue colour, impurity elements such as Al, Si, P, S, Cu and Sr, under 10 wt% in total, were detected other than the major element Ca. In the blue area, 0.04 wt% of CuO was detected, which was supposed to cause the blue colour. Vein-shaped white area showed high contents of Al, Si, P, S and Sr, and this indicated that this type of aragonite contained more impurities than Type-1 aragonite. In the Type-3 beads that contained blue dye, Al, Si, P, S, Cu and Sr were detected similarly (figure-1). However, several wt% of Cu was detected in blue dye area or around the drilled holes. This blue colour was assumedly artificially dyed, and the stones were possibly coloured in blue by hydrosoluble Cu component. Unlike the Type-1 and 2 described above, many beads contained impurity elements reaching to 20 wt% distributed on the surface layer except Ca, the major component of aragonite.
Furthermore, some stones from the Type-2 beads showing blue-green colour and the Type-3 beads containing blue dye were selected to be scratched in small area for X-ray powder diffraction analysis. The result of the Type-2 blue-green area corresponded well to the diffraction data of aragonite. In blue dye are in the Type-3 stones an extra diffraction peak was seen around 2Æ=46other than the diffraction peaks of aragonite, however, the peak was not prominent enough to be identified. Among all the beads we tested this time, the Type-2 stones concluded to resemble closely to blue aragonite from Peru with lower Sr content by spectral analysis with spectrophotometer and EDXRF chemical composition analysis. Blue aragonite from Peru generally contains several wt% to several dozens wt% of SrO. The low purity aragonite containing high content of Sr is commercially named as gVectoriteh. For further information, please contact: GAAJ Research Laboratory E-mail: ahmadjan@gaaj-zenhokyo.co.jp Website: http://www.gaaj-zenhokyo.co.jp/index-e.html |
||||||||||||||||||||||||||||||||