|
||||||||||||||||||||||||
|
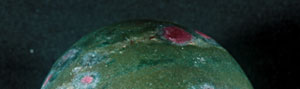
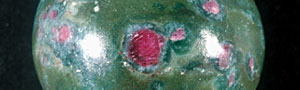
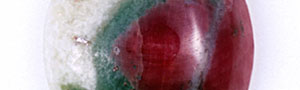
Recently we had a chance to examine ruby in a translucent to opaque rock shown in the photo. Our analysis showed that the object was ruby in muscovite (fuchsite) containing high concentration of chromium(Cr). This study describes its detail.
Sample and Analysis Two pieces of the material, one bead (47.57g) and one cabochon (7.079ct) were examined in this study. According to Mr. Minoru KAMEYAMA of Miyuki Co.Ltd., who contributed the samples, these were newly discovered in Mysory district in the south India. The samples were analysed by an X-ray analytical microscope (JSX-3600), an X-ray powder diffraction system (XRD-6000) and a Raman spectroscope (RENISHAW 1000) to distinguish each mineral phases. With the X-ray analytical microscope, the samples were measured on 90, 200, 50 and 10 points, i.e., 350 points in total, on the surface in white, green, red and brown parts of matrix respectively, using an X-ray beam converged in 50 Κm which was selected by a micro collimator. Three-dimensional chemical composition mapping that can be observed from any direction was also figured out by making the two-dimensional composition mapping in wide area of 7~7 mm. Each part of different colour was powdered to be analysed on an X-ray powder diffraction system and each variety was searched by the data obtained. Also each part of different colour in appearance was analysed by a Raman spectroscope using an Ar-ion laser of 488nm. Results of Analysis
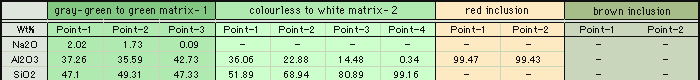
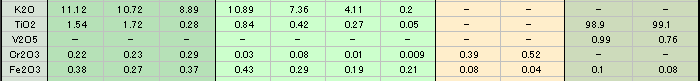
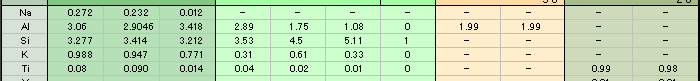
As you can see in the analysis results in Table-1, the matrix of aggregated crystal was composed of different mineral phases, one is gray-green to green and the other is colourless to white. Large part of the matrix is gray-green to green, transparent to translucent muscovite (fuchsite) containing high concentration of Cr, and has composition of Al2O3F35.59`42.73wt%ASiO2F47.4`54.0wt%AK2OF 8.89`11.12wt%ANa2OF0.09`2.02wt% within the all measurement value. It contains trace elements such as Fe, Ti, Cr, Rb and Sr, and the content of Cr2O3 reaches to 0.22`0.29wt%. It also shows high concentration of TiO2 (0.28`2.55wt%) because large amount of minute brown rutile inclusions are contained in the matrix. In green area, Cr content tends to rise and Ti content tends to reduce as the colour hue becomes brighter. From the results of compositional analysis in Table-1, its chemical formula is expressed as K0.9Na0.1(Al,Cr)3Si3O10(OH)2. This muscovite variety with high concentration of Cr is called fuchsite, among which high quality material from Minas Gerais mine in south east Brazil and Wolfefs Neck metamorphic belt in Maine, USA, can be a simulant of jadeite. White area composing a part of matrix consists of Si-rich muscovite, in which SiO2 value shows wide range of 51.89`80.89wt%. Microcrystalline quartz crystals are mixed in this matrix, which can be confirmed by data obtained by X-ray powder diffraction system. Contents of Al and K tend to reduce as Si content becomes rich. Fe and Ti of under 1wt% are contained as impurities, but Cr content is almost too low to be detected. Very few amount of Ba was detected. Red part is corundum, that is, ruby, and has wide range of Cr2O3 content 0.25`0.6wt% and low contents of Fe2O3F0.15wt% and Ga2O3F0.002wt% or lower. Other trace transition metal elements such as Ti, V and Mn were not detected. Brown crystal was rutile, containing small amount of V and Fe as impurities. Each matrix and inclusion described above can be confirmed on a microscopic Raman spectroscope. Green matrix consisted of minute crystal aggregation, which made the Raman spectrum rather weak and the identification of muscovite was difficult.
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||